What Would Be the Physical Sign That a Bone Cannot Continue Longitudinal Growth
Key Messages
-
The bony skeleton is a remarkable organ that serves both a structural function, providing mobility, support, and protection for the body, and a reservoir function, as the storehouse for essential minerals.
-
During childhood and adolescence bones are sculpted by a process called modeling, which allows for the formation of new bone at one site and the removal of old bone from another site within the same bone. This process allows individual bones to grow in size and to shift in space.
-
Much of the cellular activity in a bone consists of removal and replacement at the same site, a process called remodeling. The remodeling process occurs throughout life and becomes dominant by the time that bone reaches its peak mass (typically by the early 20s). Remodeling continues throughout life so that most of the adult skeleton is replaced about every 10 years.
-
Both genes and the environment contribute to bone health. Some elements of bone health are determined largely by genes, and errors in signaling by these genes can result in birth defects. External factors, such as diet and physical activity, are critically important to bone health throughout life, and these factors can be modified.
-
The growth of the skeleton, its response to mechanical forces, and its role as a mineral storehouse are all dependent on the proper functioning of a number of systemic or circulating hormones that respond to changes in blood calcium and phosphorus. If calcium or phosphorus are in short supply, the regulating hormones take them out of the bone to serve vital functions in other systems of the body. Too many withdrawals can weaken the bone.
-
Many things can interfere with the development of a strong and healthy skeleton. Genetic abnormalities can produce weak, thin bones, or bones that are too dense. Nutritional deficiencies can result in the formation of weak, poorly mineralized bone. Many hormonal disorders can also affect the skeleton. Lack of exercise, immobilization, and smoking can also have negative effects on bone mass and strength.
-
Osteoporosis, the most common bone disease, typically does not manifest until late in life, when bone loss begins due to bone breakdown and decreased levels of bone formation. Loss of bone mass leads to the development of structural abnormalities that make the skeleton more fragile.
The purpose of this chapter is to provide an overview of bone biology that will help the reader to understand:
-
why humans have bones;
-
how bones work;
-
how bones change during life;
-
what keeps bones healthy;
-
what causes bone disease, including the most common form, osteoporosis; and
-
the future of bone biology and what it means for preventing and treating bone disease.
While dealing with a subject that is highly technical in nature, this chapter attempts to explain bone biology in terms that a lay person can generally understand. It is intended to provide the reader with the background needed to understand the basis for some of the preventive, diagnostic, and treatment approaches related to bone disease that are discussed in detail later in this report. Those interested in a more detailed review of bone biology and bone disease can consult any of a number of recent texts (Bilezikian et al. 2001, Marcus et al. 2001, Favus 2003).
Why Do We Have Bones?
The bony skeleton is a remarkable organ that serves both a structural function—providing mobility, support, and protection for the body—and a reservoir function, as the storehouse for essential minerals. It is not a static organ, but is constantly changing to better carry out its functions. The development of the bony skeleton likely began many eons ago, when animals left the calcium-rich ocean, first to live in fresh water where calcium was in short supply, and then on dry land where weight bearing put much greater stress on the skeleton. The architecture of the skeleton is remarkably adapted to provide adequate strength and mobility so that bones do not break when subjected to substantial impact, even the loads placed on bone during vigorous physical activity. The shape or structure of bone is at least as important as its mass in providing this strength.
The skeleton is also a storehouse for two minerals, calcium and phosphorus, that are essential for the functioning of other body systems, and this storehouse must be called upon in times of need. The maintenance of a constant level of calcium in the blood as well as an adequate supply of calcium and phosphorus in cells is critical for the function of all body organs, but particularly for the nerves and muscle. Therefore, a complex system of regulatory hormones has developed that helps to maintain adequate supplies of these minerals in a variety of situations. These hormones act not only on bone but on other tissues, such as the intestine and the kidney, to regulate the supply of these elements. Thus one reason that bone health is difficult to maintain is that the skeleton is simultaneously serving two different functions that are in competition with each other. First, bone must be responsive to changes in mechanical loading or weight bearing, both of which require strong bones that have ample supplies of calcium and phosphorus. When these elements are in short supply the regulating hormones take them out of the bone to serve vital functions in other systems of the body. Thus the skeleton can be likened to a bank where we can deposit calcium or phosphorus and then withdraw them later in times of need. However, too many withdrawals weaken the bone and can lead to the most common bone disorder, fractures.
Both the amount of bone and its architecture or shape are determined by the mechanical forces that act on the skeleton. Much of this is determined genetically so that each species, including humans, has a skeleton that is adapted to its functions. However, there can be great variation within a species, so that some individuals will have strong bones and others will have weak bones, largely because of differences in their genes (Huang et al. 2003). Moreover, bone mass and architecture are further modified throughout life as these functions and the mechanical forces required to fulfill them change. In other words, bones will weaken if they are not subjected to adequate amounts of loading and weight bearing for sufficient periods of time. If they are not (such as in the weightless condition of space travel), rapid bone loss can occur. In other words, as with muscle, it is "use it or lose it" with bone as well. Conversely, the amount and architecture of the bones can be improved by mechanical loading. However, as described in Chapter 6, some types of exercise may be better than others in strengthening the skeleton.
To respond to its dual roles of support and regulation of calcium and phosphorus, as well as to repair any damage to the skeleton, bone is constantly changing. Old bone breaks down and new bone is formed on a continuous basis. In fact, the tissue of the skeleton is replaced many times during life. This requires an exquisitely controlled regulatory system that involves specialized cells that communicate with each other. These cells must respond to many different signals, both internal and external, mechanical and hormonal, and systemic (affecting the whole skeleton) and local (affecting only a small region of the skeleton). It is not surprising that with so many different tasks to perform and so many different factors regulating how the skeleton grows, adapts, and responds to changing demands, there are many ways that these processes can go astray.
How Bones Work
Bone is a composite material, consisting of crystals of mineral bound to protein. This provides both strength and resilience so that the skeleton can absorb impact without breaking. A structure made only of mineral would be more brittle and break more easily, while a structure made only of protein would be soft and bend too easily. The mineral phase of bone consists of small crystals containing calcium and phosphate, called hydroxyapatite. This mineral is bound in an orderly manner to a matrix that is made up largely of a single protein, collagen. Collagen is made by bone cells and assembled as long thin rods containing three intertwined protein chains, which are then assembled into larger fibers that are strengthened by chemical connections between them. Other proteins in bone can help to strengthen the collagen matrix even further and to regulate its ability to bind mineral. Very small changes in the shape of the bone can act on the cells inside bone (the osteocytes), which produce chemical signals that allow the skeleton to respond to changes in mechanical loading. Abnormalities in the collagen scaffold can occur as a result of a genetic disorder called osteogenesis imperfecta, while the failure of mineral deposition can be the result of rickets and osteomalacia, conditions that result in marked weakening of the skeleton (see below and Chapter 3).
To provide the body with a frame that is both light and strong, bones are hollow. The outer dense shell is called cortical bone, which makes up roughly three-quarters of the total skeletal mass. Inside the cortical shell is a fine network of connecting plates and rods called trabecular bone that makes up the remaining 25 percent (Figure 2-1). Most bones are hollow structures in which the outer cortical bone shell defines the shape of the bone. This cortical shell is essential because it provides strength, sites for firm attachment of the tendons, and muscles and protection without excessive weight. The inner trabecular network has two important functions. It provides a large bone surface for mineral exchange. In addition, trabecular bone helps to maintain skeletal strength and integrity, as it is particularly abundant in the spine and at the ends of the long bones, sites that are under continuous stress from motion and weight-bearing. Fractures are common at these sites when the bone is weakened (Kontulainen, Sievanen et al. 2003). The rods and plates of trabecular bone are aligned in a pattern that provides maximal strength without too much bulk, much in the way that architects and engineers design buildings and bridges. The shape and size of both cortical and trabecular bone can respond to different kinds of stress produced by physical activity. For example, in most people the cortex of their dominant arm is larger than that of their non-dominant arm. The difference in cortex size is even larger for tennis players and other athletes who routinely use a dominant arm in their sporting activities. Bones do not work in isolation, but rather are part of the musculoskeletal system, providing the "lever" that allows muscles to move (by pulling on the lever). Thus muscle activity is important for the normal function of the bone. When the mechanical force produced by muscle is lost—for example, in patients with muscular dystrophy or paralysis—bone mass and strength are also rapidly lost. Many bones in the skeleton also have connecting joints that provide greater flexibility of movement. These joints are sites of great mechanical stress and are subject to injury and to degeneration with aging. The most common type of joint degeneration is osteoarthritis, a painful, degenerative condition that affects the hip, knees, neck, lower back, and/or small joints of the hand. These joint diseases result from very different causes and require very different management than do bone diseases, and consequently they are not covered in this report. However it is important to recognize that the bones, joints, and muscles are the key parts of an integrated "musculoskeletal system." Problems with any one component of this system can affect the other components. Thus, weakness of the muscles can lead to loss of bone and joint damage, while degeneration of the joints leads to changes in the underlying bone, such as the bony spurs or protuberances that occur in osteoarthritis.
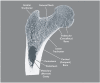
Figure 2-1
Frontal Longitudinal Midsection of Upper Femur. Source: Gray 1918.
How Bones Change Throughout Life
Throughout life, bones change in size, shape, and position. Two processes guide these changes—modeling and remodeling. When a bone is formed at one site and broken down in a different site its shape and position is changed. This is called modeling (Figure 2-2). However, much of the cellular activity in a bone consists of removal and replacement at the same site, a process called remodeling. The remainder of this section explains why and how these processes occur.

Figure 2-2
Modeling and Remodeling. Note: In modeling, osteoblast and osteoclast action are not linked and rapid changes can occur in the amount, shape, and position of bone. In remodeling, osteoblast action is coupled to prior osteoclast action. Net changes in (more...)
Why We Need Modeling and Remodeling
During childhood and adolescence bones are sculpted by modeling, which allows for the formation of new bone at one site and the removal of old bone from another site within the same bone (Seeman 2003) (Figure 2-2). This process allows individual bones to grow in size and to shift in space. During childhood bones grow because resorption occurs inside the bone while formation of new bone occurs on its outer (periosteal) surface. At puberty the bones get thicker because formation can occur on both the outer and inner (endosteal) surfaces. As people get older, resorption occurs on inner surfaces while formation occurs on outer surfaces, which can partially compensate for the loss of strength due to the thinning of the cortex. The size and shape of the skeleton follows a genetic program, but can be greatly affected by the loading or impact that occurs with physical activity. Ultimately bones achieve a shape and size that fits best to their function. In other words, "form follows function."
The remodeling process occurs throughout life and becomes the dominant process by the time that bone reaches its peak mass (typically by the early 20s). In remodeling, a small amount of bone on the surface of trabeculae or in the interior of the cortex is removed and then replaced at the same site (Figure 2-2). The remodeling process does not change the shape of the bone, but it is nevertheless vital for bone health, for a variety of reasons. First, remodeling repairs the damage to the skeleton that can result from repeated stresses by replacing small cracks or deformities in areas of cell damage. Remodeling also prevents the accumulation of too much old bone, which can lose its resilience and become brittle. Remodeling is also important for the function of the skeleton as the bank for calcium and phosphorus. Resorption (the process of breaking down bone), particularly on the surface of trabecular bone, can supply needed calcium and phosphorus when there is a deficiency in the diet or for the needs of the fetus during pregnancy or an infant during lactation. When calcium and phosphorus supplies are ample the formation phase of remodeling can take up these minerals and replenish the bank.
Modeling and remodeling continue throughout life so that most of the adult skeleton is replaced about every 10 years. While remodeling predominates by early adulthood, modeling can still occur particularly in response to weakening of the bone. Thus with aging, if excessive amounts of bone are removed from the inside, some new bone can be laid down on the outside, thus preserving the mechanical strength of the bone despite the loss of bone mass.
How Modeling and Remodeling Occur
The process of building the skeleton and continuously reshaping it to respond to internal and external signals is carried out by specialized cells that can be activated to form or break down bone. Both modeling and remodeling involve the cells that form bone called osteoblasts and the cells that break down bone, called osteoclasts (Figure 2-3). In remodeling there is an important local interaction between osteoblasts or their precursors (the cells that will develop into osteoblasts by acquiring more specialized functions—a process called differentiation) and osteoclasts or their precursors. Since remodeling is the main way that bone changes in adults and abnormalities in remodeling are the primary cause of bone disease, it is critically important to understand this process. In addition, recent research has provided exciting information about these cell interactions.
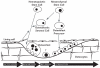
Figure 2-3
Bone Remodeling. Note: The sequence of activation, resorption, reversal, and formation is illustrated here. The activation step depends on cells of the osteoblast lineage, either on the surface of the bone or in the marrow, acting on blood cell precursors (more...)
Osteoblasts are derived from precursor cells that can also be stimulated to become muscle, fat or cartilage; however, under the right conditions these cells change (or differentiate) to form new bone, producing the collagen that forms the scaffolding or bone matrix. This calcium- and phosphate-rich mineral is added to the matrix to form the hard, yet resilient, tissue that is healthy bone. Osteoblasts lay down bone in orderly layers that add strength to the matrix. Some of the osteoblasts are buried in the matrix as it is being produced and these are now called osteocytes. Others remain as thin cells that cover the surface and are called lining cells. Osteocytes are the most numerous cells in bone and are extensively connected to each other and to the surface of osteoblasts by a network of small thin extensions. This network is critical for the ability of bone to respond to mechanical forces and injury. When the skeleton is subjected to impact there is fluid movement around the osteocytes and the long-cell extensions that provides signals to the bone cells on the surface to alter their activity, either in terms of changes in bone resorption or formation. Failure of the osteoblasts to make a normal matrix occurs in a congenital disorder of the collagen molecule called osteogenesis imperfecta. Inadequate bone matrix formation also occurs in osteoporosis, particularly in the form of osteoporosis produced by an excess of the adrenal hormones called glucocorticoid-induced osteoporosis. This form of osteoporosis differs from primary osteoporosis and most other forms of secondary osteoporosis because with glucocorticoid-induced osteoporosis inhibition of bone formation is the dominant mechanism for weakening of the skeleton.
The osteoclasts remove bone by dissolving the mineral and breaking down the matrix in a process that is called bone resorption. The osteoclasts come from the same precursor cells in the bone marrow that produce white blood cells. These precursor cells can also circulate in the blood and be available at different sites in need of bone breakdown. Osteoclasts are formed by fusion of small precursor cells into large, highly active cells with many nuclei. These large cells can fasten onto the bone, seal off an area on the surface, and develop a region of intense activity in which the cell surface is highly irregular, called a ruffled border. This ruffled border contains transport molecules that transfer hydrogen ions from the cells to the bone surface where they can dissolve the mineral. In addition, packets of enzymes are secreted from the ruffled border that can break down the matrix. Excessive bone breakdown by osteoclasts is an important cause of bone fragility not only in osteoporosis, but also in other bone diseases such as hyperparathyroidism, Paget's disease, and fibrous dysplasia (see Chapter 3). Inhibitors of osteoclastic bone breakdown have been developed to treat these disorders (see Chapter 9).
Removal and replacement of bone in the remodeling cycle occurs in a carefully orchestrated sequence that involves communication between cells of the osteoblast and osteoclast lineages (Hauge, Qvesel et al. 2001; Parfitt 2001). It is controlled by local and systemic factors that regulate bone remodeling to fulfill both its structural and metabolic functions. The activation of this process involves an interaction between cells of the osteoblastic lineage and the precursors that will become osteoclasts. What stops this process is not known, but the osteoclasts machinery clearly slows down and the osteoclasts die by a process that is called programmed cell death. Thus the amount of bone removed can be controlled by altering the rate of production of new osteoclasts, blocking their activity, or altering their life span. Most current treatments for osteoporosis work by slowing down osteoclastic bone breakdown through use of antiresorptive agents.
The activation and resorption phases are followed by a brief reversal phase (Everts, Delaisse et al. 2002). During the reversal phase the resorbed surface is prepared for the subsequent formation phase, in part by producing a thin layer of protein, rich in sugars, which is called the cement line and helps form a strong bond between the old bone and the newly formed bone.
These three phases are relatively rapid, probably lasting only 2 to 3 weeks in humans. The final phase of bone formation takes much longer, lasting up to 3 or 4 months. Thus active remodeling at many sites can weaken the bone for a considerable period of time (even if formation catches up eventually), as many defects form in the bony structure that have not yet been filled. Formation is carried out by large active osteoblasts that lay down successive layers of matrix in an orderly manner that provides added strength. The addition of minerals to the collagenous matrix completes the process of making strong bone. Any error in this complex process can lead to bone disease.
Since remodeling serves both the structural and metabolic functions of the skeleton, it can be stimulated both by the hormones that regulate mineral metabolism and by mechanical loads and local damage acting through local factors. Repair of local damage is an important function of remodeling. Over time repeated small stresses on the skeleton can produce areas of defective bone, termed micro-damage. Replacement of that damaged bone by remodeling restores bone strength. Signals for these responses are probably developed by the network of osteocytes and osteoblasts, which, through their multiple connections, can detect changes in the stress placed upon bone and in the health of the small areas of micro-damage. Factors that affect the formation, activity, and life span of osteoclasts and osteoblasts as they develop from precursor cells can affect the remodeling cycle. Drugs have been developed that act in these ways, with the goal of reducing bone loss or increasing bone formation and maintaining skeletal health.
What Keeps Bones Healthy?
Both genes and the environment contribute to bone health. Some elements of bone health (e.g., the size and shape of the skeleton) are determined largely by genes, and errors in signaling by these genes can result in birth defects. External factors, such as diet and physical activity, are critically important to bone health throughout life and can be modified. As noted above, the mechanical loading of the skeleton is essential for maintenance of normal bone mass and architecture. In addition, the skeleton needs certain nutritional elements to build tissue. Not only does the skeleton require the same nutritional elements as the rest of the body, but it also has a special requirement for large amounts of calcium and phosphorus. While adequate levels of these minerals can be obtained from the mother during pregnancy and nursing, they must come from the diet thereafter.
The growth of the skeleton, its response to mechanical forces, and its role as a mineral storehouse are all dependent on the proper functioning of a number of systemic or circulating hormones produced outside the skeleton that work in concert with local regulatory factors. The systemic hormones that affect the supply of calcium and phosphorus and the formation and breakdown of bone are listed in Table 2-1. This complex system of regulatory hormones responds to changes in blood calcium and phosphorus, acting not only on bone but also on other tissues such as the intestine and the kidney. The system is illustrated for calcium regulation in Figure 2-4. Under normal conditions only part of the dietary calcium is absorbed and some calcium is secreted into the intestinal tract so that the net amount of calcium entering the body normally is only a small proportion of dietary calcium. In healthy young adults there is calcium balance, where the amount taken in is equal to the amount excreted. The bones are constantly remodeling, but breakdown and formation are equal. The kidney filters the blood, including a large amount of calcium, but most of this is taken back into the body by the kidney cells. When calcium and/or phosphorus are in short supply, the regulating hormones take them out of the bone to serve vital functions in other systems of the body. Too many withdrawals can weaken the bone. The regulatory hormones also play critical roles in determining how much bone is formed at different phases of skeletal growth and how well bone strength and mass is maintained throughout life. For example, sex hormones and the growth hormone system described below are increased during puberty, a time of rapidly increased skeletal growth. Finally, it is important to remember that the effects of hormones and mechanical forces on the skeleton are closely linked. For example, the ability of bone to respond to mechanical loading is impaired in animals lacking the receptor for estrogen (Lee et al. 2003).
Table 2-1
Most Critical Systemic Hormones Regulating Bone.

Figure 2-4
Regulation of the Calcium Levels in the Body Fluids. Note: The extracellular fluid (ECF) calcium level is regulated not only by bone, but also by the intestine and kidney as shown in this figure. In addition to the limited absorption of calcium from the (more...)
Genes, hormones, local factors, and lifestyle all play a role in determining one's peak bone mass, a level that is typically achieved by the time an individual reaches his or her late teens or early 20s. The stronger the bones are at this time, the better able they are to deal with any withdrawals of calcium and phosphorus that are needed and with any other changes to bone that occur with aging.
What follows is a brief description of the most important regulating hormones with respect to bone health.
Calcium-Regulating Hormones
Three calcium-regulating hormones play an important role in producing healthy bone: 1) parathyroid hormone or PTH, which maintains the level of calcium and stimulates both resorption and formation of bone; 2) calcitriol, the hormone derived from vitamin D, which stimulates the intestines to absorb enough calcium and phosphorus and also affects bone directly; and 3) calcitonin, which inhibits bone breakdown and may protect against excessively high levels of calcium in the blood.
Parathyroid hormone or PTH
PTH is produced by four small glands adjacent to the thyroid gland. These glands precisely control the level of calcium in the blood. They are sensitive to small changes in calcium concentration so that when calcium concentration decreases even slightly the secretion of PTH increases. PTH acts on the kidney to conserve calcium and to stimulate calcitriol production, which increases intestinal absorption of calcium. PTH also acts on the bone to increase movement of calcium from bone to blood. Excessive production of PTH, usually due to a small tumor of the parathyroid glands, is called hyperparathyroidism and can lead to bone loss. PTH stimulates bone formation as well as resorption. When small amounts are injected intermittently, bone formation predominates and the bones get stronger (Rubin, Cosman et al. 2002). This is the basis for a new treatment for osteoporosis (see Chapter 9).
In recent years a second hormone related to PTH was identified called parathyroid hormone-related protein (PTHrP). This hormone normally regulates cartilage and bone development in the fetus, but it can be over-produced by individuals who have certain types of cancer. PTHrP then acts like PTH, causing excessive bone breakdown and abnormally high blood calcium levels, called hypercalcemia of malignancy (Stewart 2002).
Calcitriol
Calcitriol is the hormone produced from vitamin D (Norman, Okamura et al. 2002). Calcitriol, also called 1,25 dihydroxy vitamin D, is formed from vitamin D by enzymes in the liver and kidney. Calcitriol acts on many different tissues, but its most important action is to increase intestinal absorption of calcium and phosphorus, thus supplying minerals for the skeleton. Vitamin D should not technically be called a vitamin, since it is not an essential food element and can be made in the skin through the action of ultra violet light from the sun on cholesterol. Many people need vitamin D in their diet because they do not derive adequate levels from exposure to the sun. This need occurred as people began to live indoors, wear clothes, and move further north. In northern latitudes the sun's rays are filtered in the winter and thus are not strong enough to make sufficient vitamin D in the skin. Vitamin D deficiency leads to a disease of defective mineralization, called rickets in children and osteomalacia in adults. These conditions can result in bone pain, bowing and deformities of the legs, and fractures. Treatment with vitamin D can restore calcium supplies and reduce bone loss.
Calcitonin
Calcitonin is a third calcium-regulating hormone produced by cells of the thyroid gland, although by different cells than those that produce thyroid hormones (Sexton, Findlay et al. 1999). Calcitonin can block bone breakdown by inactivating osteoclasts, but this effect may be relatively transient in adult humans. Calcitonin may be more important for maintaining bone development and normal blood calcium levels in early life. Excesses or deficiencies of calcitonin in adults do not cause problems in maintaining blood calcium concentration or the strength of the bone. However, calcitonin can be used as a drug for treating bone disease.
Sex Hormones
Along with calcium-regulating hormones, sex hormones are also extremely important in regulating the growth of the skeleton and maintaining the mass and strength of bone. The female hormone estrogen and the male hormone testosterone both have effects on bone in men and women (Falahati-Nini, Riggs et al. 2000). The estrogen produced in children and early in puberty can increase bone growth. The high concentration that occurs at the end of puberty has a special effect—that is, to stop further growth in height by closing the cartilage plates at the ends of long bone that previously had allowed the bones to grow in length.
Estrogen acts on both osteoclasts and osteoblasts to inhibit bone breakdown at all stages in life. Estrogen may also stimulate bone formation. The marked decrease in estrogen at menopause is associated with rapid bone loss. Hormone therapy was widely used to prevent this, but this practice is now controversial because of the risks of increased breast cancer, strokes, blood clots, and cardiovascular disease with hormone therapy (see Chapter 9).
Testosterone is important for skeletal growth both because of its direct effects on bone and its ability to stimulate muscle growth, which puts greater stress on the bone and thus increases bone formation. Testosterone is also a source of estrogen in the body; it is converted into estrogen in fat cells. This estrogen is important for the bones of men as well as women. In fact, older men have higher levels of circulating estrogen than do postmenopausal women.
Other Important Hormones
Growth hormone from the pituitary gland is also an important regulator of skeletal growth. It acts by stimulating the production of another hormone called insulin-like growth factor-1 (IGF-1), which is produced in large amounts in the liver and released into circulation. IGF-1 is also produced locally in other tissues, particularly in bone, also under the control of growth hormone. The growth hormone may also directly affect the bone—that is, not through IGF-1 (Wang et al. 2004). Growth hormone is essential for growth and it accelerates skeletal growth at puberty. Decreased production of growth hormone and IGF-1 with age may be responsible for the inability of older individuals to form bone rapidly or to replace bone lost by resorption (Yakar and Rosen 2003). The growth hormone/IGF-1 system stimulates both the bone-resorbing and bone-forming cells, but the dominant effect is on bone formation, thus resulting in an increase in bone mass.
Thyroid hormones increase the energy production of all body cells, including bone cells. They increase the rates of both bone formation and resorption. Deficiency of thyroid hormone can impair growth in children, while excessive amounts of thyroid hormone can cause too much bone breakdown and weaken the skeleton (Vestergaard and Mosekilde 2002). The pituitary hormone that controls the thyroid gland, thyrotropin or TSH, may also have direct effects on bone (Abe et al. 2003).
Cortisol, the major hormone of the adrenal gland, is a critical regulator of metabolism and is important to the body's ability to respond to stress and injury. It has complex effects on the skeleton (Canalis and Delany 2002). Small amounts are necessary for normal bone development, but large amounts block bone growth. Synthetic forms of cortisol, called glucocorticoids, are used to treat many diseases such as asthma and arthritis. They can cause bone loss due both to decreased bone formation and to increased bone breakdown, both of which lead to a high risk of fracture (Kanis et al. 2004).
There are other circulating hormones that affect the skeleton as well. Insulin is important for bone growth, and the response to other factors that stimulate bone growth is impaired in individuals with insulin deficiency (Lu et al. 2003, Suzuki et al. 2003). A recently discovered hormone from fat cells, leptin, has also been shown to have effects on bone (Elefteriou et al. 2004, Cornish et al. 2002).
What Causes Diseases of Bone?
Maintaining a strong and healthy skeleton is a complicated process that requires having the right amount of bone with the right structure and composition in the right place. There are many things that can go wrong along the way.
Genetic abnormalities can produce weak, thin bones, or bones that are too dense. The disease osteogenesis imperfecta is caused by abnormalities in the collagen molecule that make the matrix weak and can lead to multiple fractures. In another congenital disorder, osteopetrosis, the bones are too dense because of failure of osteoclast formation or function. This failure of the remodeling process results in persistence of trabecular bone in the marrow space so that the marrow cavity may not be large enough to form red and white blood cells normally. These dense bones cannot remodel well in response to mechanical forces or micro damage and hence may be weaker and subject to fracture even though bone mass is increased. There are also other abnormalities of the genes that affect the size and shape of the skeleton and can cause deformities or abnormal growth.
Nutritional deficiencies, particularly of vitamin D, calcium, and phosphorus, can result in the formation of weak, poorly mineralized bone. In children, vitamin D deficiency produces rickets in which there is not only a marked weakness of bone and fractures but also bowing of the long bones and a characteristic deformity due to overgrowth of cartilage at the ends of the bones. In adults, vitamin D deficiency leads to a softening of the bone (a condition known as osteomalacia) that can also lead to fractures and deformities.
Many hormonal disorders can also affect the skeleton. Overactive parathyroid glands or hyperparathyroidism can cause excessive bone breakdown and increase the risk of fractures. In severe cases, large holes or cystic lesions appear in the bone, which makes them particularly fragile. A deficiency of the growth hormone/IGF-1 system can inhibit growth, leading to short stature. Loss of gonadal function or hypogonadism in children and young adults can cause severe osteoporosis due to loss of the effects of testosterone and estrogen. In addition, too much cortisol production by the adrenal gland can occur in Cushing's syndrome.
Use of glucocorticoids as medication is a common cause of bone disease. Excess glucocorticoids will stop bone growth in children and cause marked thinning of the bone in adults, often leading to fracture.
Many bone disorders are local, affecting only a small region of the skeleton. Inflammation can lead to bone loss, probably through the production of local resorbing factors by the inflammatory white cells. This process can occur around the affected joints in patients with arthritis. Bacterial infections, such as severe gum inflammation or periodontal disease, can produce loss of the bones around the teeth, and osteomyelitis can produce a loss of bone at the site of infection. This type of bone loss is due to the direct damaging effect of bacterial products as well as the production of resorbing factors by white cells. Paget's disease is a multifaceted condition in which the first change is the formation of large, highly active, and unregulated osteoclasts that produce abnormal bone resorption. The precise cause of Paget's disease is not known, but it appears to be the consequence of both genetic factors and environmental factors, possibly a viral infection. The osteoblasts try to repair this damage by increasing bone formation. However, the normal bone architecture has been disrupted, leading to weak bones and the potential for fractures and deformities (even though the bones may appear dense on an x-ray). One reason for this is that the new bone formed is disorderly, "woven" bone, which does not have the proper alignment of mineral crystals and collagen matrix. In addition, the new bone may not be in the right place to provide strength.
What Is Osteoporosis?
Osteoporosis is by far the most common bone disease. Osteoporosis is "a skeletal disorder characterized by compromised bone strength, predisposing to an increased risk of fracture" (Osteoporosis 2000). The composition of the mineral and matrix, the fine structure of the trabecular bone, the porosity of the cortical bone, and the presence of micro-fractures and other forms of damage in bone are all important in determining bone strength. Changes in the fine structure or micro-architecture of trabecular bone are particularly important since the most common fractures in osteoporosis occur at the spine, wrist, and hip, sites where trabecular bone predominates. As shown in Figure 2-5, the structure of normal trabecular bone consists of well-connected plates or broad bands that provide great strength. In individuals with osteoporosis these bands are disrupted and often become thin, weakened rods. Some of these rods are no longer connected to another piece of bone, meaning that they no longer contribute to bone strength.

Figure 2-5
Normal vs. Osteoporotic Bone. Note: These pictures, called scanning electron micrographs, are from biopsies of a normal and an osteoporotic patient. The normal bone shows a pattern of strong interconnected plates of bone. Much of this bone is lost in (more...)
Unfortunately, however, it is not possible to measure bone strength directly, or to detect changes in the micro-architecture of bone in living patients. The mass of bone, its density, and its general shape can be determined by radiographs and absorptiometry (see Chapter 8). These measures are used as "proxies" for bone strength in assessing the risk of osteoporosis today.
There are a number of different ways in which osteoporosis can develop, with the skeleton becoming more fragile and the risk of fracture increasing (Raisz and Rodan 2003). Some of the most important mechanisms that lead to skeletal fragility and fractures are listed in Table 2-2. Many people have relatively weak bones even as young adults because of their genes or because of suboptimal nutrition and lifestyle. However, fractures due to bone fragility rather than severe injury are uncommon in young adults. It is typically not until later in life that bone loss begins due to bone breakdown, a process that accelerates around the time of menopause in women. At the same time, bone formation tends to decrease with age in both men and women, typically failing to keep up with the rate of bone resorption. An imbalance between bone resorption and bone formation results in loss of bone mass, leading to the development of structural abnormalities that make the skeleton more fragile. There are a number of different combinations of increased resorption and decreased formation that can result in a weakened skeletal structure (see Figure 2-5). Each of these pathways can be involved in producing skeletal fragility at different times or sites within an individual patient. Since bone breakdown is the first step in this process, blocking bone resorption is one way to decrease bone loss and prevent fractures. It is currently the most widely used therapeutic approach in osteoporosis. Stimulation of bone formation can also reverse skeletal fragility; new therapies based on this approach have recently been developed (Chapter 9).
Table 2-2
Causes of Bone Loss and Fractures in Osteoporosis.
The Future: Where a Better Understanding of Bone Biology Can Take Us
This brief overview of the basics of bone health and disease provides a framework for the discussion of what is known about the causes, prevention, and treatment of skeletal disorders today. Many knowledge gaps remain, and it is still unclear precisely why so many people suffer fractures. Fortunately there have recently been a number of exciting new discoveries about skeletal regulation, and there are undoubtedly many more to come. These discoveries will further increase our understanding of bone health and disease.
For example, recent discoveries have shown how osteoblastic and osteoclastic cells communicate and provide signals to begin the process of resorption (Figure 2-6). The osteoblastic cells produce macrophage colony stimulating factor (M-CSF) and receptor activator of nuclear factor kappa B ligand (RANKL) (Khosla 2001), proteins that bind to receptors on the osteoclast precursors, stimulate their proliferation and differentiation, and increase osteoclast activity. Osteoblastic cells also produce a protein called osteoprotegerin that can bind RANKL and prevent it from interacting with osteoclastic cells. The hormones and local factors that stimulate bone resorption act on this system. The balance between RANKL and osteoprotegerin (OPG) production is probably critical in determining how fast bone breaks down. RANKL in bone is increased in individuals with estrogen deficiency (Eghbali-Fatourechi et al. 2003). While RANKL excess or osteoprotegerin deficiency would be expected to cause bone loss, measurements of the amounts of these proteins in circulating blood do not support this theory. OPG levels are higher and RANKL levels are lower in patients with fractures or low bone mass (Schett et al. 2004, Jorgensen et al. 2004). On the other hand, OPG or drugs that act like it by interfering with the binding of RANKL could be useful in the treatment of osteoporosis.
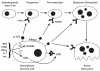
Figure 2-6
How Osteoclasts Are Formed. Note: The interaction between cells of the osteoblastic lineage and the osteoclast lineage is illustrated here. The osteoblastic cells produce several proteins that regulate osteoblast formation and activity. One is macrophage (more...)
Recently another signaling system was discovered in bone involving a receptor called lipoprotein receptor-related protein 5. Patients with over-activity in this receptor have strong bones that typically do not fracture (Boyden, Mao et al. 2002; Little, Carulli et al. 2002). Patients in whom this receptor does not function form severe osteoporosis (Gong, Slee et al. 2001). Smaller variations in the gene for this receptor may have an important influence on bone size and strength (Ferrari et al. 2004). Many other genes have also recently been identified as influencing bone mass and strength. A gene for an enzyme called lipoxygenase was recently found to affect bone mass in mice (Klein et al. 2004). Genetics studies in Iceland have shown that variants in one of the genes for bone morphogenetic proteins are associated with osteoporosis (Styrkarsdottir et al. 2003). There are also unidentified genes on specific sites on chromosomes that appear to control bone mass and architecture.
All of these new findings could ultimately lead to much better ways of determining whether or not an individual will develop a disorder of the skeleton. Enough information exists today about the causes, prevention, diagnosis, and treatment of bone diseases to increase the bone health and decrease the risk of fracture among Americans today. The goal of this report is to describe how this can be accomplished and how both personal and public health measures can promote bone health in our population.
Key Questions for Future Research
Remarkable progress in furthering our understanding of the cellular, molecular biology, and genetics of skeletal tissues in the last quarter century has provided answers to many key questions. As expected, these answers have given rise to additional research questions, as outlined below. The answers to these new questions should, in turn, lead to new approaches to diagnosis, prevention, and treatment. Thus it is important to maintain strong support for basic research, even as existing research findings are applied to the everyday practice of medicine.
-
How does the normal skeleton respond to mechanical forces and maintain the best structure?
-
How is this response lost in those individuals who develop bone disease? Local factors that contribute to this process have been identified but their specific roles are not known. In addition, there is a general understanding of bone remodeling, but there are many specific steps—in particular the reversal phase—about which little is known.
-
How precisely does estrogen maintain bone mass and strength?
-
What is the relative importance of other circulating hormones in maintaining bone health? These include not only the calcium and growth-regulating hormones, but also recently identified hormones such as leptin.
-
How do newly identified genes and proteins (e.g., the Wnt signaling pathway) that affect bone cells work?
References
-
Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, Iqbal J, Eldeiry L, Rajendren G, Blair HC, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003 Oct 17;115(2):151–62. [PubMed: 14567913]
-
Bilezikian JP, Marcus R, Levine MA, editors. The parathyroids. 2nd ed. San Diego (CA): Academic Press; 2001.
-
Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002 May 16;346(20):1513–21. [PubMed: 12015390]
-
Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci. 2002 Jun:966, 73–81. [PubMed: 12114261]
-
Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson GC, et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002 Nov;175(2):405–15. [PubMed: 12429038]
-
Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003 Apr;111(8):1221–30. [PMC free article: PMC152939] [PubMed: 12697741]
-
Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, Ogawa Y, Liu X, Ware SM, Craigen WJ, et al. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci U S A. 2004 Mar 2;101(9):3258–63. [PMC free article: PMC365777] [PubMed: 14978271]
-
Everts V, Delaisse JM, Korper W, Jansen DC, Tigchelaar-Gutter W, Saftig P, Beertsen W. The bone lining cell: Its role in cleaning Howship's lacunae and initiating bone formation. J Bone Miner Res. 2002 Jan;17(1):77–90. [PubMed: 11771672]
-
Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000 Dec;106(12):1553–60. [PMC free article: PMC381474] [PubMed: 11120762]
-
Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 5th Edition. Washington (DC): American Society for Bone and Mineral Research; 2003.
-
Ferrari SL, Deutsch S, Choudhury U, Chevalley T, Bonjour JP, Dermitzakis ET, Rizzoli R, Antonarakis SE. Polymorphisms in the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in whites. Am J Hum Genet. 2004 May;74(5):866–75. [PMC free article: PMC1181981] [PubMed: 15077203]
-
Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001 Nov 16;107(4):513–23. [PubMed: 11719191]
-
Gray H. Anatomy of the Human Body. Philadelphia: New York: Lea & Febiger; Bartleby.com; 1918. 2000 [cited 2004 Jun 9]. Available from www
.bartleby.com/107/illus247.html. -
Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res. 2001 Sep;16(9):1575–82. [PubMed: 11547826]
-
Huang QY, Recker RR, Deng HW. Searching for osteoporosis genes in the post-genome era: Progress and challenges. Osteoporos Int. 2003 Sep;14(9):701–15. [PubMed: 12904838]
-
Jorgensen HL, Kusk P, Madsen B, Fenger M, Lauritzen JB. Serum osteoprotegerin (OPG) and the A163G polymorphism in the OPG promoter region are related to peripheral measures of bone mass and fracture odds ratios. J Bone Miner Metab. 2004;22(2):132–8. [PubMed: 14999524]
-
Kanis JA, Johansson H, Oden A, Johnell O, De Laet C, Melton LJ III, Tenenhouse A, Reeve J, Silman AJ, Pols HA, Eisman JA, McCloskey EV, Mellstrom D. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004 Jun;19(6):893–99. [PubMed: 15125788]
-
Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001 Dec;142(12):5050–5. [PubMed: 11713196]
-
Klein RF, Allard J, Avnur Z, Nikolcheva T, Rotstein D, Carlos AS, Shea M, Waters RV, Belknap JK, et al. Regulation of bone mass in mice by the lipoxygenase gene Alox15. Science. 2004 Jan 9;303(5655):229–32. [PubMed: 14716014]
-
Kontulainen S, Sievanen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: A peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res. 2003 Feb;18(2):352–9. [PubMed: 12568413]
-
Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Endocrinology: Bone adaptation requires oestrogen receptor. Nature. 2003;424:389. [PubMed: 12879058]
-
Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002 Jan;70(1):11–9. Epub 2001 Dec 03. [PMC free article: PMC419982] [PubMed: 11741193]
-
Lu H, Kraut D, Gerstenfeld LC, Graves DT. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology. 2003 Jan;144(1):346–52. [PubMed: 12488363]
-
Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. Second Edition. Vol. 2. San Diego (CA): Academic Press; 2001. pp. 207–27.
-
Mundy GR, Guise TA. Hormonal control of calcium homeostasis. Clin Chem. 1999 Aug;45(8 Pt 2):1347–52. [PubMed: 10430817]
-
Norman AW, Okamura WH, Bishop JE, Henry HL. Update on biological actions of 1alpha,25(OH)(2)-vitamin D(3) (rapid effects) and 24R,25(OH)(2)-vitamin D(3). Mol Cell Endocrinol. 2002 Nov 29:197, 1–2, 1–13. [PubMed: 12431790]
-
Osteoporosis Prevention, Diagnosis, and Therapy. NIH Consensus Statement Online. 2000 March 27–29;17(1):136. [cited 2004 Mar 26]
-
Parfitt AM. The bone remodeling compartment: A circulatory function for bone lining cells. J Bone Miner Res. 2001 Sep;16(9):1583–5. [PubMed: 11547827]
-
Raisz LG, Rodan GA. Pathogenesis of osteoporosis. Endocrinol Metab Clin North Am. 2003 Mar;32(1):15–24. [PubMed: 12699290]
-
Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004 Apr 24;363(9418):1377–85. [PubMed: 15110498]
-
Rubin MR, Cosman F, Lindsay R, Bilezikian JP. The anabolic effects of parathyroid hormone. Osteoporos Int. 2002;13(4):267–77. [PubMed: 12030541]
-
Schett G, Kiechl S, Redlich K, Oberhollenzer F, Weger S, Egger G, Mayr A, Jocher J, Xu Q, Pietschmann P, et al. Soluble RANKL and risk of nontraumatic fracture. JAMA. 2004 Mar 3;291(9):1108–13. [PubMed: 14996780]
-
Seeman E. Periosteal bone formation—a neglected determinant of bone strength. N Engl J Med. 2003 Jul 24;349(4):320–3. [PubMed: 12878736]
-
Sexton PM, Findlay DM, Martin TJ. Calcitonin. Curr Med Chem. 1999 Nov;6(11):1067–93. [PubMed: 10519914]
-
Stewart A F. Hyperparathyroidism, humoral hypercalcemia of malignancy, and the anabolic actions of parathyroid hormone and parathyroid hormone-related protein on the skeleton. J Bone Miner Res. 2002 May;17(5):758–62. [PubMed: 12009005]
-
Styrkarsdottir U, Cazier JB, Kong A, Rolfsson O, Larsen H, Bjarnadottir E, Johannsdottir VD, Sigurdardottir MS, Bagger Y, Christiansen C, et al. Linkage of Osteoporosis to Chromosome 20p12 and Association to BMP2. PLoS Biol. 2003 Dec;1(3):E69. [PMC free article: PMC270020] [PubMed: 14691541]
-
Suzuki K, Miyakoshi N, Tsuchida T, Kasukawa Y, Sato K, Itoi E. Effects of combined treatment of insulin and human parathyroid hormone (1–34) on cancellous bone mass and structure in streptozotocin-induced diabetic rats. Bone. 2003 Jul;33(1):108–14. [PubMed: 12919705]
-
Vestergaard P, Mosekilde L. Fractures in patients with hyperthyroidism and hypothyroidism: A nationwide follow-up study in 16,249 patients. Thyroid. 2002 May;12(5):411–9. [PubMed: 12097203]
-
Yakar S, Rosen CJ. From mouse to man: Redefining the role of insulin-like growth factor-I in the acquisition of bone mass. Exp Biol Med (Maywood). 2003 Mar;228(3):245–52. [PubMed: 12626768]
-
Wang J, Zhou J, Cheng CM, Kopchick JJ, Bondy CA. Evidence supporting dual, IGF-I-independent and IGF-I-dependent, roles for GH in promoting longitudinal bone growth. J Endocrinol. 2004 Feb;180(2):247–55. [PubMed: 14765976]
Source: https://www.ncbi.nlm.nih.gov/books/NBK45504/
0 Response to "What Would Be the Physical Sign That a Bone Cannot Continue Longitudinal Growth"
Post a Comment